When you pick up a generic pill from the pharmacy, you expect it to work just like the brand-name version you’ve been taking. But how do we know it really does? The answer lies in something called bioequivalence-a strict, science-backed measure of how quickly and completely your body absorbs the drug. It’s not about price. It’s not about color or shape. It’s about what happens inside your bloodstream.
What Bioequivalence Really Means
Bioequivalence isn’t a marketing term. It’s a legal and scientific requirement. For a generic drug to be approved by the FDA, it must deliver the same amount of active ingredient into your blood at the same rate as the brand-name drug. This isn’t guesswork. It’s measured using two key numbers: AUC and Cmax.AUC stands for Area Under the Curve. It tells you how much of the drug your body absorbs over time-basically, the total exposure. Cmax is the highest concentration the drug reaches in your blood. That tells you how fast it gets absorbed. Both numbers must fall within a very specific range when compared to the brand-name version.
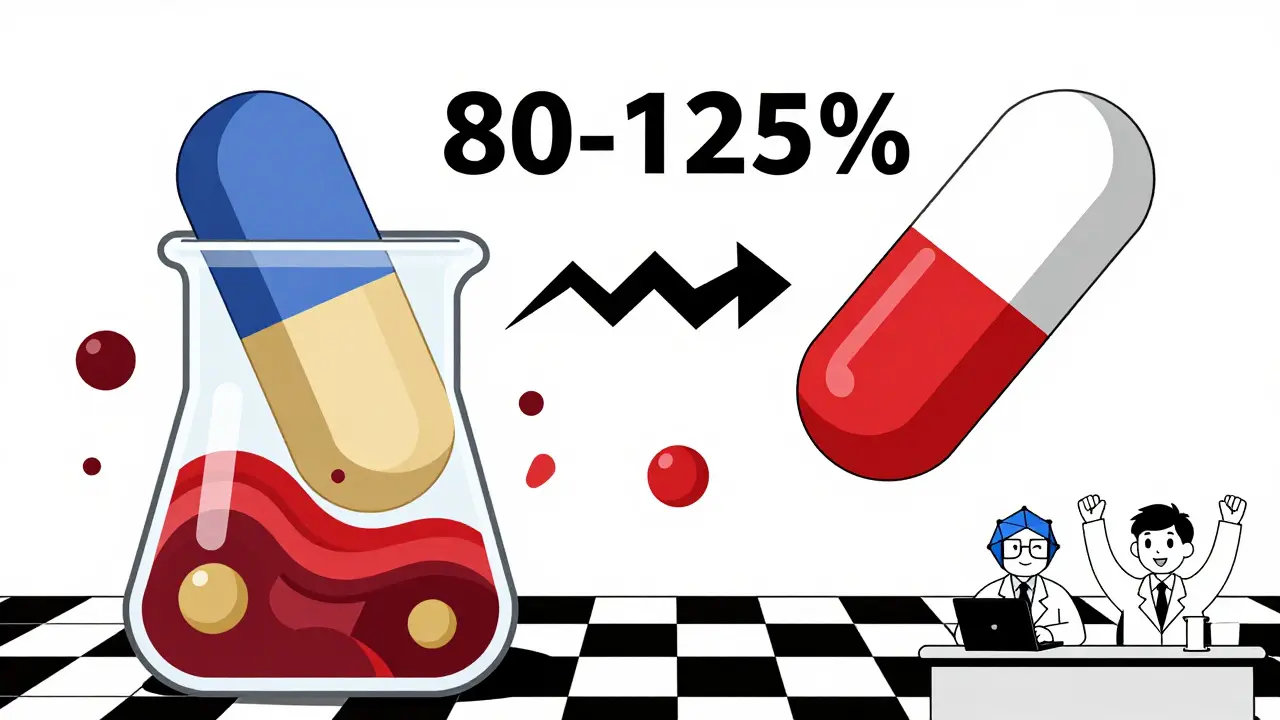
The rule? The 90% confidence interval for the ratio of generic to brand absorption must be completely between 80% and 125%. That means if the brand drug gives you 100 units of absorption, the generic must deliver between 80 and 125 units. Not 79. Not 126. Exactly within that window. And it’s not just one study. The FDA requires multiple tests on 24 to 36 healthy volunteers, using a crossover design where each person takes both the brand and generic versions, with a clean break in between.
Here’s what that looks like in real numbers: a review of over 2,000 FDA-approved bioequivalence studies found that, on average, generics differed from brand drugs by just 3.5% in AUC and 4.35% in Cmax. That’s less than the natural variation your own body shows when you take the same pill twice. Your absorption rate changes slightly from day to day due to food, stress, or even your gut bacteria. The generic drug has to perform as consistently as your own body does.
Why the 80-125% Range Isn’t a Loophole
You might hear people say, “Generics can be 20% weaker or 25% stronger.” That’s wrong. That 80-125% range isn’t about individual pills being off by 45%. It’s about the entire statistical confidence interval. Think of it like this: if you flip a coin 10 times, you might get 6 heads and 4 tails. But if you flip it 1,000 times, the result settles near 50-50. Bioequivalence studies are like flipping the coin thousands of times. The average result has to land so close to the brand that there’s no meaningful difference.The FDA doesn’t approve a generic unless the entire 90% confidence interval fits inside 80-125%. That means even the lowest possible absorption rate for the generic, based on the study data, still can’t fall below 80% of the brand. And the highest possible rate still can’t exceed 125%. In practice, most generics land right at 98-102%. The system is designed to catch outliers before they reach you.
Dissolution Isn’t the Same as Absorption
You might read about generic pills dissolving slower or faster in lab tests. That’s true. A 2014 study found that more than half of the generic drugs tested had different dissolution rates than their brand-name counterparts. Some generic nifedipine took much longer to break down. Some amoxicillin dissolved faster. That sounds alarming-but it doesn’t matter.Dissolution is just the first step. It’s what happens in a test tube. Absorption is what happens in your body. A pill can dissolve slowly but still be absorbed completely because your stomach and intestines are better at pulling the drug into your blood than a lab beaker. The FDA doesn’t care how fast the pill breaks apart in water. They care how fast it shows up in your blood. And if the blood levels match, the pill works.
That’s why a generic that dissolves slower can still be bioequivalent. The body compensates. The drug stays in the gut longer and gets absorbed anyway. The system is built to account for real biology, not just lab conditions.
What About Narrow Therapeutic Index Drugs?
Some drugs don’t have room for error. Warfarin, digoxin, phenytoin, and levothyroxine are in this group. A tiny change in blood levels can cause a clot, a seizure, or a thyroid crisis. For these, the FDA tightens the rules. The acceptable range shrinks from 80-125% to 90-111% for AUC. That’s a much tighter leash.That’s why pharmacists are trained to be extra careful when switching patients on these drugs. Even if the generic is approved, switching from one brand to another generic-or even between batches of the same generic-can sometimes trigger a reaction in sensitive patients. That’s not because the drug is bad. It’s because the body has adapted to a very specific level. A shift of 5% might be fine for most drugs, but not for these.
Doctors often keep patients on the same generic manufacturer for these drugs. If you’re stable on one brand of generic levothyroxine, your doctor may prefer you stay on it-not because the others are unsafe, but because consistency matters more than cost here.
Why Do Some People Say Generics Don’t Work?
You’ve probably heard stories. “I switched to generic bupropion and felt awful.” “My thyroid meds stopped working after the pharmacy changed the label.” These aren’t made up. But they’re not proof that generics are inferior.Drugs.com analyzed over 1,200 patient reviews and found that 12% reported perceived differences, with levothyroxine and bupropion topping the list. But when you look at the science, the numbers don’t match. A 2023 meta-analysis of 47 studies with nearly 10,000 patients found no difference in outcomes between generic and brand-name cardiovascular drugs. The FDA has documented only 12 cases of potential therapeutic failure among more than 14,000 approved generics since 2008. That’s a failure rate of 0.08%.
So why the disconnect? Placebo effect plays a role. If you believe generics are “weaker,” your brain might interpret normal side effects as the drug not working. Also, generics can look different-color, size, shape-because trademark laws forbid them from copying the brand’s appearance. That visual change can trigger anxiety. One study showed patients reported more side effects when told they were switching to a generic-even when they weren’t.
And then there’s the issue of switching between generic manufacturers. Even if both are FDA-approved, different fillers, coatings, or manufacturing processes can cause subtle differences in how the drug behaves in your body. That’s why some doctors prefer you stick with one generic brand once you’ve found one that works.
The Real-World Impact
Generics make healthcare affordable. In the U.S., they make up 90% of all prescriptions but only 23% of drug spending. In 2023, the generic market was worth $135.7 billion. Without bioequivalence standards, this system wouldn’t exist. No one would trust generics. No pharmacy would stock them. No insurer would cover them.Patients save an average of $1,000 a year just by using generics. For people on chronic meds, that’s life-changing. A 2019 report found that new patients abandoned brand-name drugs at 266% higher rates than generics, mostly because of cost. The average copay for a generic is under $20. For a brand, it’s often over $100.
And it’s not just the U.S. The European Medicines Agency uses the same 80-125% standard. Japan uses a slightly tighter range for some drugs. This isn’t a U.S.-only rule. It’s global science.
What You Should Do
If you’re taking a generic drug and feel fine-stick with it. There’s no reason to switch unless your doctor says so. If you’re switching from brand to generic and notice a change in how you feel, talk to your doctor. Don’t assume it’s the drug. It could be stress, sleep, diet, or even a new supplement.For narrow therapeutic index drugs, ask your pharmacist: “Is this the same manufacturer as before?” If you’re stable, try to stay on the same one. Don’t let a pharmacy change it without telling you.
And if you’re worried about quality, check the FDA’s Orange Book. It lists every approved generic and its therapeutic equivalence rating. An “A” rating means it’s approved as equivalent. A “B” rating means there’s a known issue-rare, but it happens. Your pharmacist can tell you which one you’re getting.
The bottom line? Generics work. They’re not cheaper because they’re worse. They’re cheaper because they don’t need to pay for advertising, branding, or patent lawsuits. The science behind them is solid. The data is overwhelming. And the people who rely on them-millions of them-get better because of them.
What’s Next for Generic Drugs?
The FDA is now exploring new ways to prove bioequivalence without always testing on people. Using computer models and lab data, they hope to reduce the need for human studies for simpler drugs. That could speed up approvals and lower costs even more.For complex drugs-like inhalers, creams, or injectables-new testing methods are being developed. These aren’t as easy to measure with blood levels, so the FDA is creating special rules. But the goal stays the same: make sure the generic does exactly what the brand does, no more, no less.
By 2027, the FDA aims to approve 90% of generic applications in 10 months or less. That’s faster than ever. And with no signs of loosening the 80-125% standard, you can be confident that the next generic you pick up will work just like the brand.
Are generic drugs as effective as brand-name drugs?
Yes. Generic drugs must meet the same strict bioequivalence standards as brand-name drugs. The FDA requires that the amount of active ingredient absorbed into your bloodstream must be within 80-125% of the brand’s absorption rate. In practice, most generics differ by less than 5%. Thousands of studies and decades of real-world use confirm they work the same way.
Why do some people feel different on generic medications?
Perceived differences are often due to psychological factors, like expecting a cheaper drug to be less effective. Changes in pill size, color, or shape can also trigger anxiety. Sometimes, switching between different generic manufacturers-even if both are FDA-approved-can cause minor fluctuations in how the drug is absorbed. This is most common with narrow therapeutic index drugs like levothyroxine or warfarin. If you notice a change, talk to your doctor before switching back.
Can I trust generics for serious conditions like heart disease or thyroid disorders?
Yes, with some exceptions. For most conditions, generics are just as safe and effective. For drugs with a narrow therapeutic index-like warfarin, digoxin, phenytoin, and levothyroxine-the FDA uses tighter standards (90-111%) and recommends sticking with the same manufacturer once you’re stable. Studies show no difference in outcomes for heart disease drugs between brand and generic. Always follow your doctor’s advice.
How does the FDA ensure generic drugs are safe after they’re on the market?
The FDA monitors all drugs-brand and generic-through its Adverse Event Reporting System. If reports suggest a problem, they can require new studies or pull the product. Since 2008, only 12 out of more than 14,000 approved generics have raised potential safety concerns. That’s a failure rate of 0.08%. The system works.
What’s the difference between dissolution and absorption?
Dissolution is how quickly a pill breaks down in water during lab tests. Absorption is how quickly your body takes the drug into your bloodstream. A generic pill might dissolve slower in a test tube but still be absorbed just as well in your gut. The FDA only cares about absorption. That’s why a drug can have different dissolution rates but still be approved as bioequivalent.
Why do generics look different from brand-name drugs?
U.S. trademark laws prevent generics from looking exactly like brand-name drugs. That means different colors, shapes, sizes, or even flavors. These differences don’t affect how the drug works. They’re only there to avoid legal issues. The active ingredient and its absorption rate are what matter-and those are tightly controlled.


December 18, 2025 AT 18:16 PM
Generics saved my life. I’m on three of them and have been for years. No issues. No side effects. Just steady, reliable relief. If you’re worried, talk to your pharmacist. They know the stuff inside out.
December 18, 2025 AT 19:18 PM
MY DOCTOR SWITCHED ME TO A GENERIC AND I FELT LIKE I WAS DRIFTING THROUGH A FOG FOR TWO WEEKS. I THOUGHT I WAS GOING CRAZY. THEN I SWITCHED BACK AND BOOM - I WAS MYSELF AGAIN. THIS ISN’T JUST ‘PLACEBO.’ THIS IS REAL.
December 19, 2025 AT 17:54 PM
That 80-125% range is often misunderstood. It’s not that generics can be 20% weaker - it’s that the *entire statistical distribution* of absorption across subjects must fall within that window. Most are within 95-105%. The FDA doesn’t cut corners.
December 20, 2025 AT 23:47 PM
Let’s be real - the reason generics work is because the FDA holds them to the same standard as brands. No fluff. No marketing spin. Just hard data from human trials. And the fact that over 90% of prescriptions are generics and we haven’t had a mass health crisis says everything. People panic over pill color. The science doesn’t care.
Some folks blame generics when their sleep’s off or they’re stressed. But if you’re on a narrow index drug like levothyroxine? Stick with the same maker. Consistency matters. Not because generics are bad - because biology is finicky.
The dissolution vs absorption confusion? Classic. Pills break down differently in a beaker than in your gut. Your intestines aren’t a lab. They’re a living, breathing, enzyme-rich miracle machine. If the blood levels match? It works. End of story.
And yes, there are outliers. But 12 cases out of 14,000 approved generics? That’s less than a single plane crash in a decade of air travel. We don’t ban planes because of rare crashes. We fix the system. Same here.
Stop letting big pharma scare you with vague anecdotes. The data is on the side of your wallet and your health. Generics are not ‘cheap.’ They’re smart. And they’re saving millions of people from financial ruin while keeping them alive.
Next time you see a $15 generic vs a $120 brand? Don’t hesitate. Trust the science. Trust the regulators. And thank the FDA for doing their job.
December 21, 2025 AT 01:26 AM
So you’re telling me the government says it’s fine for a drug to be 20% weaker? That’s not science. That’s corporate collusion. Big Pharma makes the brand, then owns the generic too. Same factory. Same people. Same profit margin. You think they’d risk a recall? They’re not worried. They’re laughing.
December 21, 2025 AT 20:37 PM
OMG I switched to generic bupropion and I cried for 3 days straight. I thought I was depressed again but it was the pill. My soul felt hollow. I went back to brand and now I’m smiling. This isn’t placebo - it’s betrayal.
December 22, 2025 AT 10:35 AM
For anyone in Canada or Europe - same rules apply. EMA uses 80-125%. Japan uses 90-111% for some drugs. This isn’t a U.S. scam. It’s global science. If you’re skeptical, check the WHO’s guidelines. They back it too.
And if you’re worried about fillers? Yeah, generics use different binders. But those don’t affect absorption. They’re just there to hold the pill together. Think of it like different shoe brands - same sole, different laces.
Also, if your pharmacy switches your generic without telling you? Speak up. Pharmacists are required to notify you. If they don’t, ask why. Knowledge is power.
December 22, 2025 AT 22:32 PM
Been on generic warfarin for 8 years. No clots. No bleeds. My INR’s steady. My doctor says stick with the same batch. I do. I don’t care what it looks like. I care that my blood flows right.
December 24, 2025 AT 13:58 PM
I used to be super skeptical too. Then I started reading the FDA’s reports. The data is insane. Most generics are within 2-3% of the brand. That’s less than the variation you get from drinking coffee before taking it. I stopped stressing. Now I save $800 a year. No regrets.
December 26, 2025 AT 07:33 AM
It is, of course, utterly preposterous to suggest that a pharmaceutical product manufactured under the aegis of regulatory oversight could possibly exhibit therapeutic equivalence to its branded counterpart - particularly when one considers the inherent variability in excipient composition, dissolution kinetics, and bioavailability profiles across disparate manufacturing facilities. One might even posit that the very notion of bioequivalence is a neoliberal construct designed to commodify health outcomes under the guise of fiscal prudence.
And yet - and yet - one cannot help but observe that the aggregate clinical data, peer-reviewed meta-analyses, and post-marketing surveillance reports consistently demonstrate no statistically significant divergence in outcomes. How curious. How... convenient.
Perhaps the real issue lies not in the drug itself, but in the psychological conditioning of the patient, who has been conditioned by decades of advertising to equate cost with efficacy - and therefore, by extension, to perceive inferiority where none exists. The placebo effect, in this context, is not a flaw - it is a feature of the system.
One wonders whether the FDA’s 80-125% confidence interval is not a scientific standard, but a political compromise - a threshold engineered to satisfy both corporate interests and public demand for affordability. One might even argue that the margin is deliberately wide enough to permit marginal variation - variation that, in aggregate, yields sufficient profit for manufacturers while remaining just below the threshold of regulatory alarm.
And yet - and yet - the data holds. The blood levels match. The patients stabilize. The system works. How strange. How profoundly, unsettlingly, effective.
December 27, 2025 AT 07:31 AM
Used to hate generics. Thought they were sketchy. Then I got hit with a huge insurance hike and had no choice. Switched to generic metformin. No side effects. No weirdness. Just... normal. Turns out my body didn’t care what the pill looked like. Only that it worked.
December 29, 2025 AT 03:39 AM
They don’t want you to know this - but generics are often made in the same factories as the brand names. Same machines. Same chemists. Same quality control. The only difference? The label. And the price tag. You’re paying for the logo. Not the medicine.
December 30, 2025 AT 07:53 AM
Let’s talk about the 90% CI. It’s not about mean values - it’s about the entire distribution. The FDA requires the lower bound of the 90% confidence interval to be ≥80% and the upper bound ≤125%. This ensures that even the 5th percentile of absorption in the population doesn’t fall below therapeutic thresholds. That’s not luck. That’s precision engineering.
And yes - some generics have different excipients. But excipients are tested for bioinertness. They’re not active ingredients. They’re scaffolding. Your body doesn’t care if it’s microcrystalline cellulose or pregelatinized starch - it cares about the API concentration in plasma. That’s what matters.
Also - the 3.5% AUC difference? That’s less than intra-individual variability caused by circadian rhythm, gastric pH, or recent food intake. You’re more variable than the generic.
December 30, 2025 AT 18:19 PM
I’ve been on generic levothyroxine for 5 years. My TSH is perfect. I feel great. I don’t care what it looks like. I care that I’m alive and functional. Thanks to generics, I can afford to keep taking it. Don’t let fear stop you from saving your life.
December 31, 2025 AT 16:21 PM
Is bioequivalence a scientific construct - or a social contract? We measure absorption via AUC and Cmax - but are these metrics sufficient to capture the totality of therapeutic effect? What of tissue distribution? What of receptor binding kinetics? What of epigenetic modulation over time? The FDA’s model reduces a living biological system to two statistical parameters - a reductionist triumph of quantification over complexity.
And yet - the system works. Millions live. Millions thrive. The paradox is not in the data - but in our refusal to reconcile the elegance of the model with the messiness of the human body.
Perhaps the real triumph isn’t that generics match brands - but that we’ve built a system where imperfect measurements, imperfect biology, and imperfect humans still achieve near-perfect outcomes. That’s not science. That’s civilization.
December 31, 2025 AT 20:47 PM
Wow. I came here thinking generics were sketchy. Now I’m just impressed. The science is way more rigorous than I thought. I’ll stick with mine. And maybe stop judging pills by their color.
December 31, 2025 AT 23:27 PM
This is the most reassuring thing I’ve read all year. Thank you for laying it out so clearly. I’m telling my mom about this.